Part:BBa_K3332052
J23100-RBS-INPNC-GOX-terminator
We anchored GOX protein onto membranes through INPNC to catalyze the reaction of degradating glyphosate to form glyoxalic acid and AMPA. We use K880005 to construct the expression system and anchor GOX on the surface of E.coli.
Biology
Ice nucleoprotein is an anchor protein from Pseudomonas syringae. It can anchor its passenger protein to the cell membrane. N and C terminal of Ice nucleoprotein, which is named after INPNC, can also anchor passenger protein fused with it to the cell membrane.
GOX, also known as EcAKR4-1, is found in Echinochloa colona. It can decompose glyphosate into AMPA and glyoxylic acid. GOX is fused at N terminal with INPNC so that iNap can be displayed on the surface of E. coli. [1][2][3]
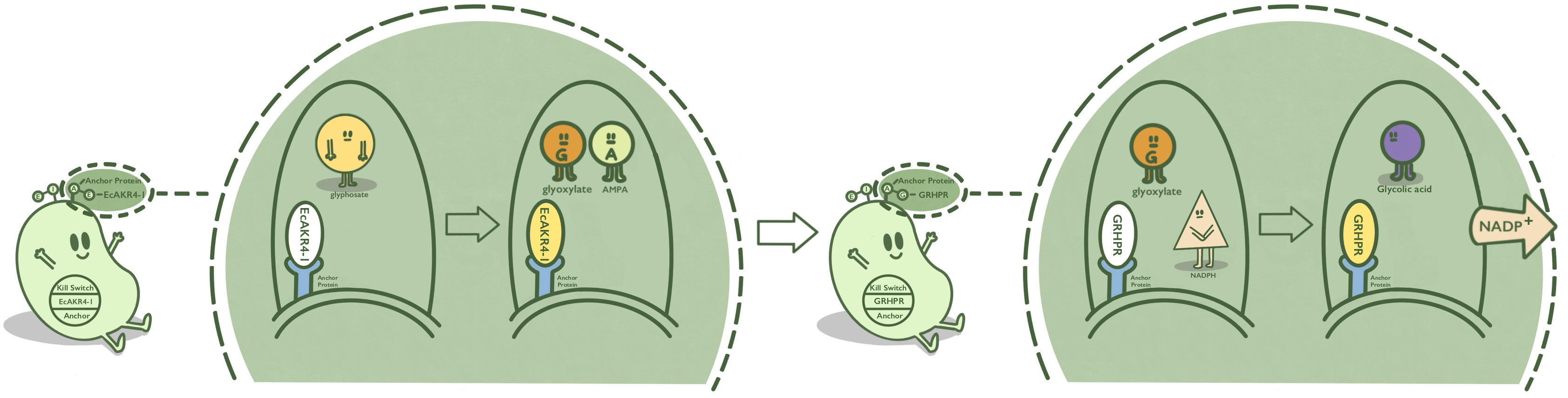
- Fig 1. Mechanism of GOX on the surface of E. coli
Usage
Here, we used BBa_K880005 to construct the expression system and demonstrated the effect of INPNC-GOX on the surface of E. coli. We obtained the composite part BBa_K3332052 and transformed the constructed plasmid into E. coli BL21 (DE3) to verify its expression. The positive clones were cultivated.

- Fig 2. Gene circuit of INPNC-GOX
Characterization
1. Identification
After receiving the synthesized DNA, PCR was done to certify that the plasmid was correct, and the experimental results were shown in figure3.

- Fig 3. DNA gel electrophoresis of PCR products of INPNC-GOX-pSB1C3
2. The proof of expression
We used J23100 promoter to highly express INPNC-GOX in E. coli in our composite part BBa_K3332052. In order to verify whether our recombinant plasmid works, we use membrane protein extraction kit to get membrane protein. Then, our target bands are observed through SDS-PAGE and the experimental results are shown in figure 4. Notably, in INPNC-GOX lane we could see our target protein band which don’t show up in controls.

- Fig 4. SDS-PAGE of membrane protein extraction products of INPNC-GOX-pSB1C3
3. Ability of degrading NADPH
After transforming glyphosate into glyoxylic acid with the function of GOX, we use GRHPR, a glyoxylate reductase from human liver, to reduce glyoxylic acid. GRHPR can convert glyoxylic acid when NADPH is consumed as cofactor. NADPH is a suitable target compound that can be detected by the signal of OD340. And when NADPH is consumed, OD340 declines.
We mixed glyphosate solution, NADPH solution, purified GRHPR protein dissolved in Tris-HCl(pH=7.5) and bacteria solution carrying INPNC-GOX. Then, we immediately detected OD340 by using TECAN® Infinite M200 Pro to see the effect of GOX fused with anchor protein.
When using INPNC-GOX bacteria solution, we successfully found OD340 decrease as time went on. When using bacteria carrying J23100-RBS(BBa_K880005) and bacteria carrying GOX-Histag, we can see the decrease is less than the INPNC-GOX samples. The results prove that INPNC-GOX can be displayed on the surface and convert glyphosate as normal, which is shown in figure 5.

- Fig 5. OD340-Time curve of three fusion protein of GOX and anchor protein
References
- ↑ Pan L, Yu Q, Han H, et al. Aldo-keto Reductase Metabolizes Glyphosate and Confers Glyphosate Resistance in Echinochloa colona[J]. Plant Physiol, 2019, 181(4): 1519-1534.
- ↑ Van Bloois E, Winter R T, Kolmar H, et al. Decorating microbes: surface display of proteins on Escherichia coli[J]. Trends Biotechnol, 2011, 29(2): 79-86.
- ↑ http://2016.igem.org/Team:TJUSLS_China
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1330
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 466
Illegal NgoMIV site found at 1549
Illegal AgeI site found at 1619
Illegal AgeI site found at 1696 - 1000COMPATIBLE WITH RFC[1000]
| None |
